Abstract
Introduction: The final events of the activated clotting cascade include the conversion of fibrinogen to fibrin and stabilization of the growing fibrin clot by factor XIII (FXIII). A missense variant in the alpha chain of fibrinogen, α-fibrinogen (FGA) Thr312Ala (rs6050, NM_000508.3:c.991A>G, NP_000499.1:p.[Thr331Ala]), has been previously shown to interfere with FXIII-dependent cross-linking, leading to stiffer blood clots. It is believed this pathophysiology underlies the increased risk of venous thromboembolism (VTE) associated with the variant. The Thr312Ala variant has been shown to be significantly associated with VTE among White, Non-Hispanic populations. The only investigation of the variant in a Black population (Rasmussen-Torvik et al 2007) found no significant relationship between Thr312Ala and VTE, but the authors cite the relatively small number of participants included in the analysis as a possible reason for a null finding. We investigated the association between FGA Thr312Ala and VTE in a case-control study enrolling a relatively large racially diverse study population.
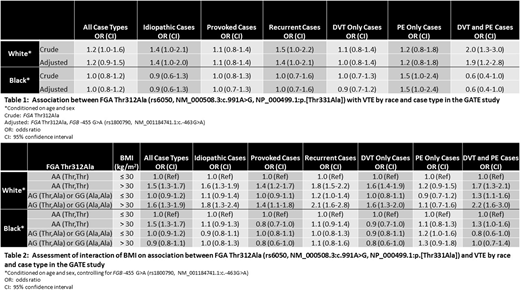
Methods: Conditional logistic regression was used to assess the association between FGA Thr312Ala and VTE among enrollees in the Genetic Attributes and Thrombosis Epidemiology (GATE) study, a case-control study enrolling 1,042 VTE cases and 1,213 controls with no history of VTE, frequency-matched on age, sex, and race. Crude models conditioned on the matching factors, and adjusted models conditioned on the matching factors and controlled for patient characteristics found to be associated with both case/control status and FGA Thr312Ala genotype. Among all patient characteristics evaluated, the β-fibrinogen (FGB) promoter region variant -455 G>A (rs1800790, NM_001184741.1:c.-463G>A) was the only patient characteristic associated with both case/control status and FGA Thr312Ala genotype. Potential effect modification was assessed using likelihood ratio tests.
Results: Among 660 White controls and 528 White cases in the GATE study, carrying at least one FGA rs6050 G (Ala) allele was associated with increased odds of VTE compared to not carrying a G allele (odds ratio (OR) and 95% confidence interval (CI) 1.2 [1.0-1.5]) in a crude model. This association was strongest when restricting to n=205 idiopathic (unprovoked) cases, n=125 recurrent cases, or n=112 cases exhibiting both deep vein thrombosis (DVT) and pulmonary embolism (PE) (Table 1). This association was modified by obesity status, with the effect among obese enrollees being multiplicative (Table 2). In contrast, there was no association between carrying at least one FGA rs6050 G (Ala) allele and VTE among 553 Black controls and 514 Black cases in the GATE study (OR and 95% CI 1.0 [0.8-1.2]) when assessing the association among all cases (Table 1). However, the odds of VTE was higher among Black participants carrying the G allele compared to those not carrying the allele when restricting to n=103 PE only cases (OR and 95% CI 1.5 [1.0-2.4]). The association between FGA Thr312Ala and VTE did not appear to be modified by obesity status among Black participants (Table 2).
Conclusions: These results indicate that the FGA Thr312Ala (rs6050, NM_000508.3:c.991A>G, NP_000499.1:p.[Thr331Ala]) polymorphism is associated with VTE among White participants in the GATE study, with the strongest effect seen in participants exhibiting both DVT and PE. However, the association among Black participants appears limited to PE, indicating further exploration of genetic risk factors for VTE in Black populations is warranted in order to better understand genetic risk factors for all types of VTE in this population.
Payne:Bayer: Other: treatment product donation; Bioverativ: Other: treatment product donation; Novo Nordisk: Other: treatment product donation; Shire: Other: treatment product donation; Genentech: Membership on an entity's Board of Directors or advisory committees. Patel:Daiichi Sankyo: Other: DSMB member.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal